ELSIセミナー
On the Origins of Life: RNA Life's First Biopolymer?
- スピーカー
- Matthew W. Powner (University College London)
- 日付
- December 9, 2014
- 時間
- 15:15
- 場所
ELSI Building - 104 Communication Room

A plausible abiotic chemical route to the canonical nucleotides is a major goal for Origins of Life research. A chemoselective, prebiotically plausible synthesis of activated canonical pyrimidine ribonucleotides and steps towards divergent pyrimidine and purine ribonucleotide synthesis will be outlined. Furthermore, natural RNA is a homogeneously ligated polymer, however, ribonucleotides have a linkage ambiguity (5'-3' versus 5'-2') at each nucleotide. A chemical solution to RNA linkage heterogeneity and ribozyme heterogeneity tolerance will both be presented.
Key words: Origins of Life, Systems Chemistry, Ribozymes, Multicomponent Reactions, RNA
Understanding the Origin of Life is one the most fundamental challenges facing modern science. 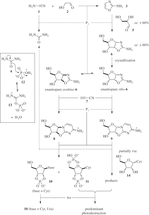 Chemistry must inevitably play a significant role in elucidating the plausible pathways through which core biomolecules can self-assemble into viable cells. Although extant life consists of an intriguingly small constellation of closely related molecules the function of the individual biological components cannot be appreciated in isolation. Therefore, it must also follow that understanding the generation (and function) of biochemical species at the Origins of Life will require a concerted and, ideally, unified investigation. Specifically, we investigate chemical divergence and convergence in abiogenesis of the essential molecular components of biology. Particularly, we concentrate on nucleotides and their precursors and, importantly, their interactions within complex systems. Nucleotides are the cornerstone of all biology and the linchpin of information transfer and inheritance. They are involved in all universally conserved biological processes - so much so that RNA genes are ubiquitously used define phylogenetic trees (i.e. the interspecies - Darwinian -
Chemistry must inevitably play a significant role in elucidating the plausible pathways through which core biomolecules can self-assemble into viable cells. Although extant life consists of an intriguingly small constellation of closely related molecules the function of the individual biological components cannot be appreciated in isolation. Therefore, it must also follow that understanding the generation (and function) of biochemical species at the Origins of Life will require a concerted and, ideally, unified investigation. Specifically, we investigate chemical divergence and convergence in abiogenesis of the essential molecular components of biology. Particularly, we concentrate on nucleotides and their precursors and, importantly, their interactions within complex systems. Nucleotides are the cornerstone of all biology and the linchpin of information transfer and inheritance. They are involved in all universally conserved biological processes - so much so that RNA genes are ubiquitously used define phylogenetic trees (i.e. the interspecies - Darwinian -
distance between organisms). However, the fundamental chemical reactivity that could have led to the "molecular biologist's dream"⎯⎯a pool of homochiral nucleotides⎯⎯remains an unmet scientific challenge.
 A plausible abiotic chemical route to the canonical nucleotides is a major goal in Origins of Life research. Some of our recent work to understand the intrinsic chemical requirements for abiotic nucleotide synthesis in water will be presented.1,2 This work includes a chemoselective, prebiotically plausible route to activated canonical pyrimidine ribonucleotides. Furthermore, steps towards a divergent pyrimidine and purine ribonucleotide synthesis will be demonstrated.3,4 Exploiting a one-pot multicomponent reaction leading to stereoselective and regiospecific purine precursors glycosylation, whilst concurrently furnishing known pyrimidine ribonucleotide precursors.
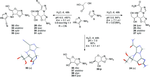
A plausible abiotic chemical route to the canonical nucleotides is a major goal in Origins of Life research. Some of our recent work to understand the intrinsic chemical requirements for abiotic nucleotide synthesis in water will be presented.1,2 This work includes a chemoselective, prebiotically plausible route to activated canonical pyrimidine ribonucleotides. Furthermore, steps towards a divergent pyrimidine and purine ribonucleotide synthesis will be demonstrated.3,4 Exploiting a one-pot multicomponent reaction leading to stereoselective and regiospecific purine precursors glycosylation, whilst concurrently furnishing known pyrimidine ribonucleotide precursors. ![]() Natural RNA is ribonucleotide polymer linked homogeneously by a 5'-3'-phosphodiester backbone. However, ribonucleotides have a linkage ambiguity (5'-3' versus 5'-2') at every phosphodiester bond. Ostensibly this linkage heterogeneity must be addressed in the absence of sophisticated enzymatic catalysis for the effective abiotic synthesis of RNA.
Natural RNA is ribonucleotide polymer linked homogeneously by a 5'-3'-phosphodiester backbone. However, ribonucleotides have a linkage ambiguity (5'-3' versus 5'-2') at every phosphodiester bond. Ostensibly this linkage heterogeneity must be addressed in the absence of sophisticated enzymatic catalysis for the effective abiotic synthesis of RNA. ![]() An acyl-transfer cascade that provides a (purely chemical) solution to the synthesis of RNA with linkage homogeneity will be described.5 Finally, the experimental demonstration of ribozyme catalysis in heterogeneous 5'-3'/5'-2' RNA will be presented.6 The ability of ribozymes to tolerate relatively high levels of non-natural backbone ligation will be presented as a novel functional selection pressure that could have operated at the Origins of Life.
An acyl-transfer cascade that provides a (purely chemical) solution to the synthesis of RNA with linkage homogeneity will be described.5 Finally, the experimental demonstration of ribozyme catalysis in heterogeneous 5'-3'/5'-2' RNA will be presented.6 The ability of ribozymes to tolerate relatively high levels of non-natural backbone ligation will be presented as a novel functional selection pressure that could have operated at the Origins of Life.
References
1) Powner, M. W.; Gerland, B.; Sutherland, J. D. Nature 2009, 495, 239.
2) Powner, M. W.; Sutherland, J. D. Angew. Chem. Int. Ed. 2010, 49, 4641.
3) Powner, M. W.; Sutherland, J. D.; Szostak, J. W. J. Am. Chem. Soc. 2010, 132, 16677.
4) Powner, M. W.; Zheng, S-L.; Szostak, J. W. J. Am. Chem. Soc., 2012, 134, 13889.
5) Bowler, F. R. et al. Nature Chem. 2013, 5, 383.
6) Engelhart, A. E.; Powner, M. W.; Szostak, J. W. Nature Chem. 2013, 5, 390.