Research Highlights
Building artificial cells
Assembling the essential components of living systems within simple cell-like structures could create artificial life sooner than we might expect
"I want to make life in the laboratory," says Yutetsu Kuruma, who does not see his goal as a distant dream. Many researchers are racing to achieve a prototype self-sustaining artificial cell, and Kuruma expects the first will be created within the next five years.
Kuruma and colleagues at the Earth-Life Science Institute (ELSI) and the University of Tokyo have taken important steps toward a breakthrough which will enhance understanding of how real cells work and to create systems that could be useful in medicine and industry. They have developed techniques to incorporate functioning protein molecules inside artificial membranes.
All cells are bounded by a fatty "lipid" membrane carrying a variety of embedded protein molecules (see image). Membranes form an essential barrier between the inside and outside of a cell. But the barrier must be selectively permeable, and membrane proteins choreograph which chemicals are allowed in and out. The proteins also control the interactions among neighboring cells.
In a paper published in Nature Protocols, Kuruma describes a cell-free system for producing proteins that are normally found within cell membranes1. Named the PURE system, it is composed of 36 enzymes and highly purified components of the protein-making machinery within cells. A related paper in Angewandte Chemie describes how Kuruma and his colleagues have synthesized a crucial protein-guiding membrane channel in the PURE system and placed it into artificial membranes2. Once embedded in the membrane, this channel guides other proteins to become incorporated properly into the membrane.
Taken together, this method offers a significantly improved and more efficient way to create artificial cell-like structures with proteins being placed and then correctly formed within their membranes. Using genetic engineering technology, it should also be possible to make membrane proteins to order, including versions not found in nature.
"This could be very useful for modeling cellular processes that are difficult to understand using real cells," says Kuruma. Engineering specific mutations in the genes of proteins of medical interest, for example, could help researchers understand a protein's role in disease and devise new therapies to treat that disease.
Artificial cells might also be used as targeted drug-delivery systems. They may even shed light on key steps involved in the origin of life itself, when the simplest possible cells presumably developed spontaneously.
The next step for Kuruma is to try to coax his cell-like structures to produce their own lipid molecules so that they can make new membranes for themselves. This will be crucial for artificial cells to develop the ability to grow and eventually divide and multiply.
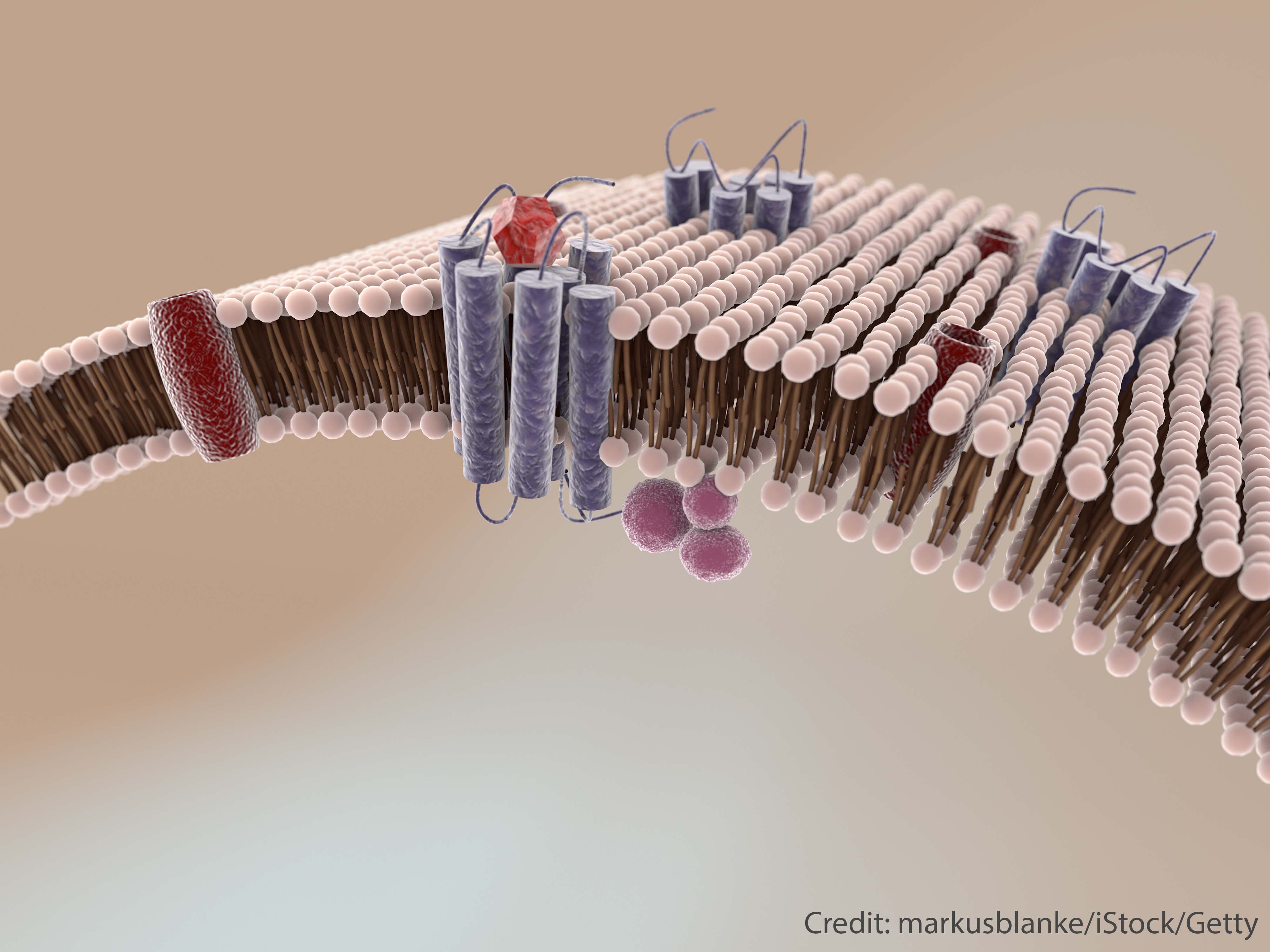
Making cell membranes with protein molecules embedded within them is a crucial step towards building artificial cell-like life. The brown-beige structures represent membrane lipid molecules and proteins are represented as blue-red structures.
References
- Kuruma, Y. & Ueda, T. The PURE System for the cell-free synthesis of membrane proteins. Nature Protocols, 10, 1328-1344, (2015).
- Matsubayashi, H., Kuruma, Y. & Ueda, T. In Vitro Synthesis of the E. coli Sec Translocon from DNA. Angewandte Chemie International Edition, 53, 7535-7538 (2014).